Soap is an indispensable part of daily life, essential for personal hygiene, household cleaning, and industrial applications. Its widespread use underscores its effectiveness in removing dirt, grease, and pathogens. But how does soap work? This article aims to provide a detailed explanation of the scientific principles governing soap’s cleaning abilities. By examining its chemical composition, mechanisms, and production, we’ll uncover the processes that make soap a cornerstone of cleanliness.
Chemical Structure and Origins of Soap

What is soap, chemically speaking? Soap is a salt formed from fatty acids and an alkali, such as sodium hydroxide or potassium hydroxide. Its molecular structure is amphiphilic, featuring a hydrophilic (water-attracting) head and a hydrophobic (water-repelling) tail. This dual nature is fundamental to how does soap work. The hydrophilic head, typically a carboxylate group, interacts with water, while the hydrophobic tail, a long hydrocarbon chain, binds to oils and greases.
Historically, soap dates back to around 2800 BCE in Mesopotamia, where fats were boiled with wood ash, a natural alkali source. The Roman Empire later refined this into a widespread practice, using animal fats and plant ashes. Today, modern soap production employs triglycerides—fats or oils—from sources like tallow or coconut oil, reacted with lye. This reaction yields soap and glycerin, a valuable byproduct.
The amphiphilic structure enables soap to bridge water and non-polar substances, a property unchanged since its inception. How does a soap work with such a simple composition? Its effectiveness lies in the molecular arrangement, allowing it to disrupt and remove non-water-soluble contaminants. This balance of polarity distinguishes soap from mere water or detergents, making it uniquely suited for cleaning. Advances in formulation have expanded its applications, yet the core chemistry remains a testament to its enduring utility. Understanding this structure is key to appreciating soap’s role in cleanliness across millennia.
The Cleaning Mechanism of Soap
How does soap work to clean? The amphiphilic nature of soap molecules enables three connected cleaning processes which lead to its effectiveness. The combination of soap with water and a contaminated surface leads to the simultaneous binding of dirt and oils and microorganisms through specific processes. The following section analyzes these specific steps.
Adsorption and Dissolution: Hydrophilic and Hydrophobic Interactions
The first step of soap cleaning starts with adsorption which allows soap molecules to bind with contaminants. The oil or grease molecules penetrate the hydrophobic ends of soap molecules while the hydrophilic ends maintain their position in water. The interaction between water and non-polar substances breaks their cohesive bonds which enables them to dissolve in the aqueous solution. How does the soap work here? The soap molecules create a bridge between water and lipids which allows water to remove substances that cannot be dissolved by water alone.
Micelle Formation: Structure and Function
The increasing soap concentration leads to the formation of micelles which serve as essential cleaning structures. The hydrophobic tails of a micelle form an inner cluster that holds oil or dirt particles while the hydrophilic heads remain exposed to water. The encapsulation process creates a solution that holds contaminants in suspension to stop their redeposition. How does a soap work through micelles? The micellar structure helps stabilize incompatible substances which enables their removal during rinsing operations needed to combat tough grease.
Emulsification: Mixing Oils with Water for Easy Rinsing
Emulsification completes the cleaning action. The small droplets of dispersed oil remain stable in water due to soap’s emulsifying action. The suspension state allows oils to stay within the water phase which makes their removal process easier. The cleaning mechanism of soap for complex stains involves what steps? Through emulsification water becomes capable of removing lipids which normally stay attached to surfaces. Through adsorption and micelle formation and emulsification soap utilizes its molecular properties to effectively clean by removing various types of contaminants.
The Saponification Process: How Soap Is Made
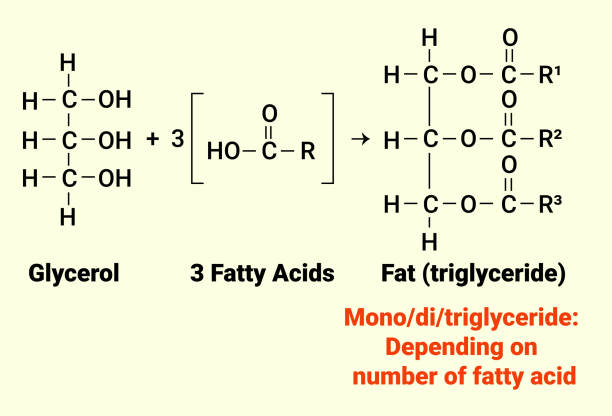
Soap production hinges on saponification, a chemical reaction between triglycerides and an alkali. Triglycerides, derived from animal fats or plant oils like palm or olive, consist of glycerol bonded to three fatty acid chains. When combined with a strong base—typically sodium hydroxide for solid soap or potassium hydroxide for liquid—the alkali hydrolyzes these esters. The result? Soap molecules and glycerol.
The process begins with precise measurements of fat and lye, heated and mixed until a thick, homogenous “trace” forms. This mixture is then poured into molds, where saponification completes over hours or days, depending on conditions. After curing—often weeks for bar soaps—the product hardens, neutralizing excess alkali for safe use. How does soap work without this reaction? It doesn’t; saponification creates the amphiphilic structure essential for cleaning.
Industrial methods refine this further, controlling temperature and additives like fragrances or moisturizers. Small-scale producers might use cold-process techniques, retaining natural glycerin, while large manufacturers opt for hot-process methods to accelerate curing. The choice of fat influences the soap’s properties—coconut oil yields a hard, bubbly bar, while olive oil produces a gentler, creamier lather. This versatility, rooted in a straightforward reaction, explains soap’s adaptability across applications, from artisanal bars to mass-produced liquids.
Benefits and Limitations of Soap in Cleaning
Soap’s benefits are well-documented. It excels at removing oils, dirt, and organic debris, leveraging its amphiphilic structure to disrupt and suspend contaminants. How does soap work to clean so effectively? Its micelles and emulsification properties allow it to address lipid-based soils that water alone cannot shift. Additionally, soap exhibits mild antimicrobial activity, reducing bacterial loads on skin or surfaces, a feature critical in hygiene.
Its affordability and accessibility enhance its appeal. Available in countless formulations—bars, liquids, or foams—soap suits diverse needs, from handwashing to laundry. Environmentally, traditional soaps biodegrade more readily than some synthetic detergents, aligning with sustainable practices. I recall a study showing soap’s efficacy against grease outpacing many alternatives, a testament to its design.
However, limitations exist. In hard water, rich in calcium and magnesium ions, soap forms insoluble salts, reducing lather and leaving residues—think bathtub rings. How does the soap work in such conditions? Less efficiently, as these precipitates hinder its action. Against enveloped viruses or resilient bacteria, soap’s impact is limited, requiring complementary sanitizers for full pathogen control. These constraints highlight the need for context-specific use, balancing soap’s strengths with its chemical boundaries.
Discover Oully: Pioneers in Innovative and Eco-Friendly Soap Making

We’ve spent over a decade perfecting soap at Oully. Our five product lines—soap, bath bombs, cosmetics and so on—reach more than 20 countries worldwide. With a daily output exceeding 100,000 units, our production capacity is unmatched. Our FDA-approved soaps deliver thorough cleansing, lasting lather, and skin-nourishing benefits, showcasing our dedication to excellence. We offer a seamless one-stop service, from formulation to delivery. Our approach ensures every bar cleans effectively while staying eco-friendly. Curious about our process? Visit our site to explore our innovative range today.
















